“The whole world thought it was impossible, but I was determined to give it a try”
Professor Mathias Uhlén, a pioneer in Swedish life science, and professor Sophia Hober, a global expert in protein binders, are behind one of Sweden’s most exported products: an antibody purification column utilizing an engineered bacterial protein.
So, what is the actual innovation? There is a bacterium that has a naturally occurring protein that binds strongly to antibodies, protein A from Staphylococcus Aureus. This protein can be used to purify antibodies from humans, animals and cultured cells. But the bacterial protein is sensitive and is destroyed when used on an industrial scale. The innovation consists in producing a designed variant which retains its properties but is stable enough to be used industrially.
A protein’s function and sensitivity are both completely dependent on its building blocks, so fixing the sensitivity issues destroys the function, in theory. Mathias Uhlén had studied the protein and its binding properties for a long time. “The whole world thought it was impossible, but I was determined to give it a try.” says Mathias Uhlén. Throughout a decade of experimentations led by Sophia Hober, they together managed to identify and successfully replace the sensitive parts of the protein. Sophia Hober adds; ”We were convinced from the start that we could improve it. Protein A is a quite stable protein to start with, so the question was really how far we could push it.”
To succeed with the stability improvements, Sophia Hober first had to de-stabilize the protein. “Usually, experiments to test a protein’s stability may take weeks. But by intentionally de-stabilizing the protein, we could speed the experiments up to minutes.”, Hober explains.
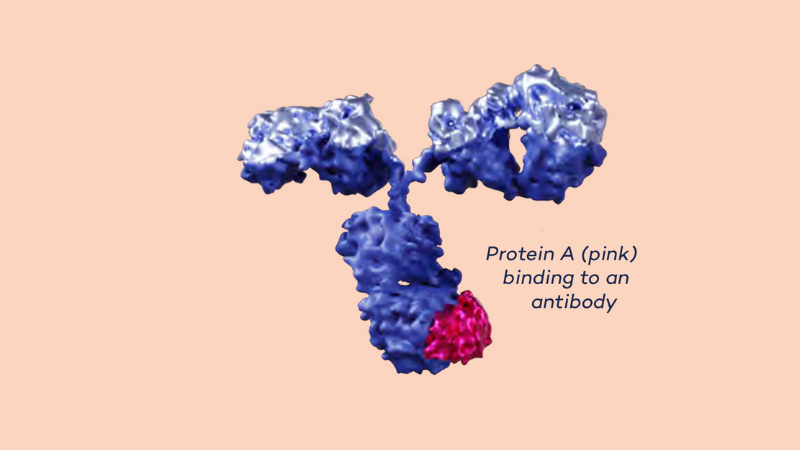
The method and the engineered protein was patented1 and the product MabSelectTM SuRe was launched in 2005. “The timing was fantastic.”, Mathias Uhlén explains. “This was a time when antibodies as medicine had just been shown to work, for example to treat a particularly aggressive form of breast cancer. Many pharmaceutical companies changed their manufacturing lines in order to be able to produce these biological medicines, which is an extremely demanding process. The purity required for an antibody to be used in humans could only be achieved with our method on an industrial scale.”
Mathias Uhlén says that due to regulatory requirements for a uniform production chain, methods in a process are rarely changed. When new producers enter an area, they prefer to choose already approved sub-components for their production line, instead of testing unproven solutions. “This meant that MabSelectTM SuRe was implemented globally and is still being used. The product simply works.” Sophia Hober adds: “Even though many other protein binders have been developed, the antibody is and will remain the gold standard biological therapeutical molecule. The market for this product will remain for a long time.”
50 years ago, technology development was a less well-regarded area of biological research, and the term “Swedish science” was used as a slight to someone who developed methods instead of solving biological problems. “On the contrary, we should be proud of this,” says Mathias Uhlén. “Technology development is only becoming more important, and much of the research that takes place in Sweden is technology- and data-driven. It is therefore fantastic that Sweden is at the forefront of the life science field in an international perspective.”
1) Patent EP 1 123 389 B1 “A method of affinity separation and ligands for use therein”, Uhlén and Hober 1999 and Patent EP 3 249 047 A2 “Mutated protein”, Hober, 2003 (among many patents)
2) In the photo: Mathias Uhlén and Sophia Hober Photo Jenny Öhman, Copyright NLS Media Group AB Photo Peter Asplund, Copyright KTH-Royal Institute of Technology
3) This text was first published in the Life Science Barometer 2025.





